Artificial Photosynthesis
Artificial photosynthesis is one of the newer ways researchers are exploring to capture the energy of sunlight reaching earth.
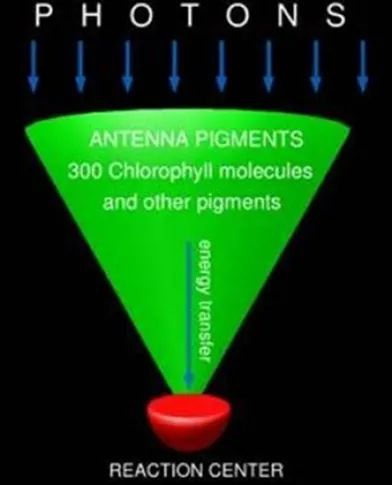
Photosynthesis:
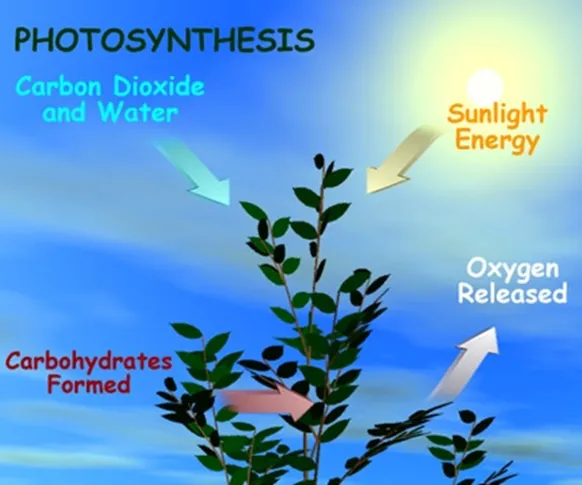
Photosynthesis is the conversion of sunlight, carbon dioxide, and water into usable fuel and it is typically discussed in relation to plants where the fuel is carbohydrates, proteins, and fats. Using only 3 percent of the sunlight that reaches the planet, plants collectively perform massive energy conversions, converting just over 1,100 billion tons of CO2 into food sources for animals every year.
Photovoltaic Technology:
This harnessing of the sun represents a virtually untapped potential for generating energy for human use at a time when efforts to commercialize photovoltaic–cell technology are underway. Using a semiconductor–based system, photovoltaic technology converts sunlight to electricity, but in an expensive and somewhat inefficient manner with notable shortcomings related to energy storage and the dynamics of weather and available sunlight.
Artificial Photosynthesis:
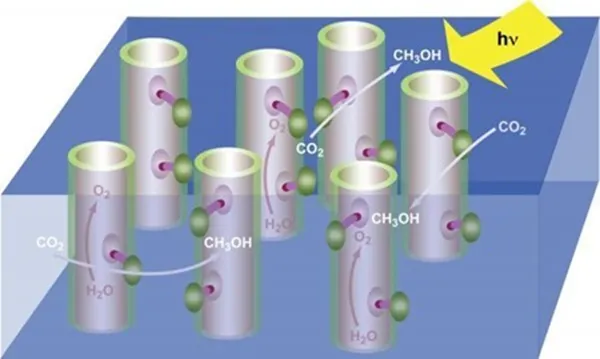
Two things occur as plants convert sunlight into energy:
● Sunlight is harvested using chlorophyll and a collection of proteins and enzymes, and
● Water molecules are split into hydrogen, electrons, and oxygen.
These electrons and oxygen then turn the CO2 into carbohydrates, after which oxygen is expelled.
Rather than release only oxygen at the end of this reaction, an artificial process designed to produce energy for human use will need to release liquid hydrogen or methanol, which will in turn be used as liquid fuel or channeled into a fuel cell. The processes of producing hydrogen and capturing sunlight are not a problem. The challenge lies in developing a catalyst to split the water molecules and get the electrons that start the chemical process to produce the hydrogen.
There are a number of promising catalysts available, that, once perfected, could have a profound impact on how we address the energy supply challenge:
● Manganese directly mimics the biology found in plants.
● Titanium Dioxide is used in dye-sensitized cell.
● Cobalt Oxide is very abundant, stable and efficient as a catalyst
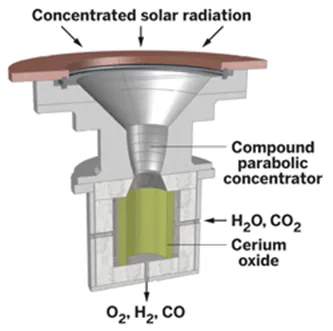
Artificial Photosynthesis Operation:
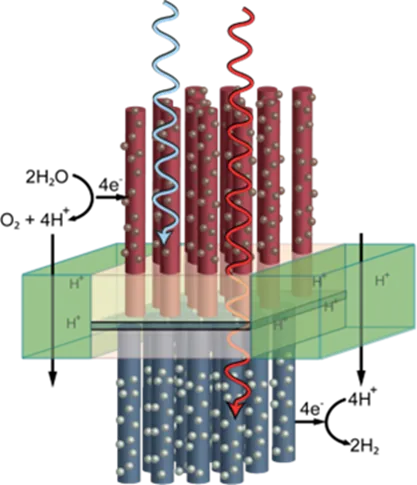
Under the fuel through artificial photosynthesis scenario, nano tubes embedded within a membrane would act like green leaves, using incident solar radiation (H³) to split water molecules (H2O), freeing up electrons and oxygen (O2) that then react with carbon dioxide (CO2) to produce a fuel, shown here as methanol (CH3OH). The result is a renewable green energy source that also helps scrub the atmosphere of excessive carbon dioxide from the burning of fossil fuels.
History:
Plants use organic compounds that need to be continuously renewed. Researchers are looking for inorganic compounds that catalyze the needed reactions and are both efficient and widely available.
The research has been significantly boosted by the application of nano technology. It’s a good example of the step wise progress in the scientific world.
Studies earlier in the decade showed that crystals iridium efficiently drove the reduction of CO2, but iridium is extremely rare so technology that required its use would be expensive and could never be used on a large scale.
Cobalt crystals were tried. They worked, and cobalt is widely available, but the original formulations weren’t at all efficient.
Things changed with the introduction of nano technology.
The main point is that this unique approach increasing appears to be feasible. It has the advantage of harnessing solar energy in a form that can be stored and used with greater efficiency than batteries and it is at least carbon neutral.