Properties of air
Air is characterised by very low cohesion, i.e. the forces between the air molecules are negligible in the operating conditions usual in pneumatics. Like all gases, air therefore does not have a specific form. It changes its shape with the least application of force and occupies the maximum space available to it.
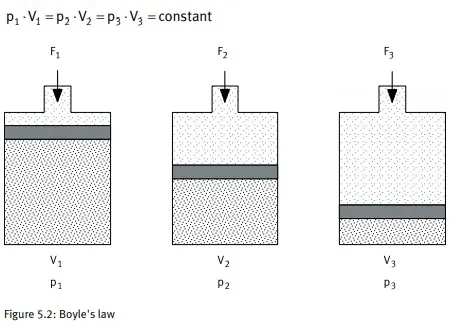
Boyle's law
Air can be compressed and attempts to expand. Boyle's law describes these properties as follows: the volume of a fixed amount of gas is inversely proportional to the absolute pressure at constant temperature, or to put it another way, the product of volume and absolute pressure is constant for a fixed amount of gas.

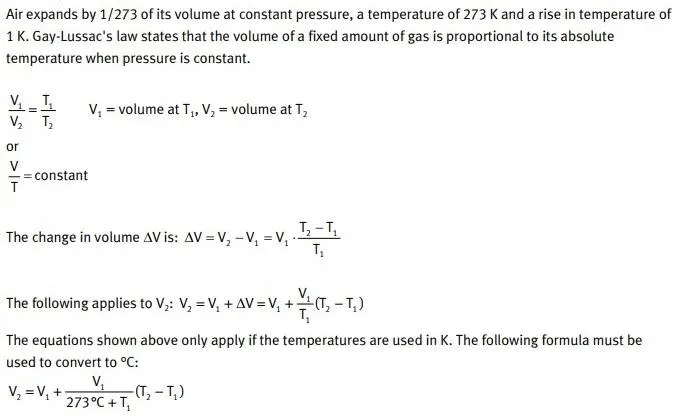
Gay-Lussac’s Law
General gas equation
Fulfilling the basic laws is the general gas equation that states: with a fixed amount of gas, the product of pressure and volume divided by the absolute temperature is constant
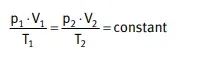
From this general gas law can be derived the above-mentioned laws, when one of the three factors p, V or T remains constant.
• Pressure p constant - isobaric change
• Volume V constant - isochoric change
• Temperature T constant - isothermal change