Electromagnetic radiation is composed of oscillating electric and magnetic fields that have the ability to transfer energy through space. The energy propagates as a wave, such that the crests and troughs of the wave move in vacuum at the speed of 299,792,458 metres per second. The many forms of electromagnetic radiation appear different to an observer; light is visible to the human eye, while X rays and radio waves are not.
The distance between successive crests in a wave is called its wavelength. The various forms of electromagnetic radiation differ in wavelength. For example, the visible portion of the electromagnetic spectrum lies between 4 × 10−7 and 8 × 10−7 metre (1.6 × 10−5 and 3.1 × 10−5 inch): red light has a longer wavelength than green light, which in turn has a longer wavelength than blue light. Radio waves can have wavelengths longer than 1,000 metres, while those of high-energy gamma rays can be shorter than 10−16 metre, which is one-millionth of the diameter of an atom. Visible light and X rays are often described in units of angstroms or in nanometres. One angstrom (abbreviated by the symbol Å) is 10−10 metre, which is also the typical diameter of an atom. One nanometre (nm) is 10−9 metre. The micrometre (μm), which equals 10−6 metre, is often used to describe infrared radiation.
The decomposition of electromagnetic radiation into its component wavelengths is fundamental to spectroscopy. Evolving from the first crude prism spectrographs that separated sunlight into its constituent colours, modern spectrometers have provided ever-increasing wavelength resolution. Large-grating spectrometers are capable of resolving wavelengths as close as 10−3 nanometre, while modern laser techniques can resolve optical wavelengths separated by less than 10−10 nanometre.
The frequency with which the electromagnetic wave oscillates is also used to characterize the radiation. The product of the frequency (ν) and the wavelength (λ) is equal to the speed of light (c); i.e., νλ = c. The frequency is often expressed as the number of oscillations per second, and the unit of frequency is hertz (Hz), where one hertz is one cycle per second. Since the electromagnetic spectrum spans many orders of magnitude, frequency units are usually accompanied by a Latin prefix to set the scale of the frequency range.
An isolated atom can be described in terms of certain discrete states called quantum states. Each quantum state has a definite energy associated with it, but several quantum states can have the same energy. These quantum states and their energy levels are calculated from the basic principles of quantum mechanics. For the simplest atom, hydrogen, which consists of a single proton and a single electron, the energy levels have been calculated and tested to an uncertainty of better than one part in 1011, but for atoms with many electrons, the accuracy of the calculations may not be much better than a few percent of the energy of the levels.
Atomic energy levels are typically measured by observing transitions between two levels. For example, an atom in its lowest possible energy state (called the ground state) can be excited to a higher state only if energy is added by an amount that is equal to the difference between the two levels. Thus, by measuring the energy of the radiation that has been absorbed by the atom, the difference in its energy levels can be determined. The energy levels are identical for atoms of the same type; allowed energies of a particular atom of silver are equal to those for any other atom of the same isotope of silver.
Other isolated systems, including molecules, ions (charged atoms or molecules), and atomic nuclei, have discrete allowed energies. The analysis of these simple systems is carried out with techniques that are analogous to those that were first applied to simple atomic spectra. More complex structures, such as clusters of atoms, and bulk condensed matter, such as solids and liquids, also have energy levels describable by quantum mechanics. The energy levels in these complex systems, however, are so closely spaced that they smear into a continuous band of energies. Transitions between these bands allow researchers to discern many important properties of a given material. The location and properties of the energy states are often referred to as the electronic structure of the material. By comparing spectroscopic measurements to quantum mechanical calculations based on an assumed model of the material, one can use knowledge of a material’s electronic structure to determine its physical structure.
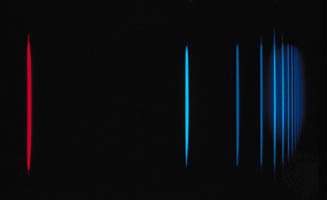
If an atom in its ground state is given some amount of energy so that it is promoted to an excited state, the atom will release that extra energy spontaneously as it moves back into lower states, eventually returning to the ground state. For an isolated atom, the energy is emitted as electromagnetic radiation. The emitted energy E equals the upper-state energy minus the lower-state energy; this energy is usually carried by a single quantum of light (a photon) having a frequency ν in which photon energy (E) is equal to a constant times the frequency, E = hν, where h, Planck’s constant, equals 6.626 × 10−34 joule second. This relationship determines the frequencies (and wavelengths, because λ = c/ν) of light emitted by atoms if the energies of the states are known. Conversely, the relationship allows the energy states of an atom to be determined from measurements of its frequency or wavelength spectrum. The analysis of the discrete wavelengths emitted or absorbed by an atom or molecule was historically carried out using prism or grating spectrometers; because of the appearance of the separated light in these instruments, these discrete wavelengths are sometimes called spectral lines.
The basis for analytical spectroscopy is the discovery, made in 1859 by the German physicist Gustav R. Kirchhoff, that each pure substance has its own characteristic spectrum. Another German physicist, Joseph von Fraunhofer, repeating more carefully an earlier experiment by a British scientist, William Wollaston, had shown in 1814 that the spectrum of the Sun’s electromagnetic radiation does not grade smoothly from one colour to the next but has many dark lines, indicating that light is missing at certain wavelengths because of absorption. These dark lines, sometimes called Fraunhofer lines, are also collectively referred to as an absorption spectrum. The spectra of materials that were heated in flames or placed in electric-gas discharges were studied by many scientists during the 18th and 19th centuries. These spectra were composed of numerous bright discrete lines, indicating that only certain wavelengths were present in the emitted light. They are called brightline, or emission, spectra.
Although the possibility that each chemical element has a unique characteristic spectrum had been considered by numerous investigators, the early studies were hampered by the difficulty of obtaining relatively pure substances. Any sample could contain impurities that would result in the simultaneous production of many spectra. By using carefully purified substances, Kirchhoff demonstrated characteristic spectra and initiated the technique of spectroscopic analysis of the chemical composition of matter. The technique was applied by Kirchhoff and his colleague the German chemist Robert Bunsen in 1861 to the analysis of the Sun’s electromagnetic spectrum and the identification of the chemical elements in the Sun.
Before the 20th century, there was no theory that could satisfactorily explain the origin of the spectra of the elements or the reason why different elements have different spectra. The quantitative understanding of the elemental spectra needed the development of a fundamentally new physical theory, and the spectra of the simplest atoms played the key role in the development of this theory. Many of the major developments in 20th-century physics were motivated by an ever-increasing accuracy in the measurement of the spectra of the hydrogen atom; highlights include the discovery in 1885 by the Swiss scientist Johann J. Balmer that the frequency spectrum of hydrogen followed a simple numerical pattern, later revised by the Swedish physicist Johannes R. Rydberg and given in modern notation as 1/λ = RH (1/22 − 1/n2), where RH is the so-called Rydberg constant for hydrogen. In 1913 the Danish physicist Niels Bohr presented the first theoretical model that could give quantized energy levels that were in quantitative agreement with measurements of the hydrogen spectrum.
Despite the success of the Bohr theory in describing the hydrogen spectrum, the theory failed badly when applied to the next simplest atom, helium, which contains two electrons. It was also incapable of predicting the likelihood of transitions between energy levels. In 1925–26 a new theory that could explain the discrete, quantum nature of the spectra was developed by the German physicists Werner Heisenberg and Erwin Schrödinger. This theory, known as quantum mechanics, was extended by the Austrian-born Swiss physicist Wolfgang Pauli, the German physicist Max Born, and others. It has been remarkably successful in describing the spectra of complex atoms, ions, simple molecules, and solids.
As the spectral lines of the hydrogen atom were measured with increased accuracy, greater demands were placed on the theoretical understanding of atomic spectra. The British physicist Paul A.M. Dirac combined quantum mechanics with the special theory of relativity in 1928 to describe particles moving close to the speed of light. His formulation of relativistic quantum mechanics provided an explanation for the so-called fine structure of the hydrogen spectrum. At still higher resolution, two energy levels of the hydrogen atom in the first excited state were predicted by Dirac’s theory to be exactly the same. In 1947, the American physicists Willis Lamb and Robert Retherford discovered that the levels actually differ by roughly 109 hertz. In contrast, the transition frequency between the ground state and the first excited states was calculated as approximately 2.5 × 1015 hertz. Two American physicists, Richard Feynman and Julian Schwinger, and a Japanese physicist, Shinichirō Tomonaga, developed yet another refinement to quantum mechanics to explain this measurement. The theory, known as quantum electrodynamics (QED), had its foundations in the discoveries of Dirac, Heisenberg, and Pauli. It is a complete description of the interaction of radiation with matter and has been used to calculate the energy levels of the hydrogen atom to an accuracy of better than 1 part in 1011. No other physical theory has the ability to predict a measurable quantity with such precision, and, as a result of the successes of quantum electrodynamics, the theory has become the paradigm of physical theories at the microscopic level.
Spectroscopy is used as a tool for studying the structures of atoms and molecules. The large number of wavelengths emitted by these systems makes it possible to investigate their structures in detail, including the electron configurations of ground and various excited states.
Spectroscopy also provides a precise analytical method for finding the constituents in material having unknown chemical composition. In a typical spectroscopic analysis, a concentration of a few parts per million of a trace element in a material can be detected through its emission spectrum.
In astronomy the study of the spectral emission lines of distant galaxies led to the discovery that the universe is expanding rapidly and isotropically (independent of direction). The finding was based on the observation of a Doppler shift of spectral lines. The Doppler shift is an effect that occurs when a source of radiation such as a star moves relative to an observer. The frequency will be shifted in much the same way that an observer on a moving train hears a shift in the frequency of the pitch of a ringing bell at a railroad crossing. The pitch of the bell sounds higher if the train is approaching the crossing and lower if it is moving away. Similarly, light frequencies will be Doppler-shifted up or down depending on whether the light source is approaching or receding from the observer. During the 1920s, the American astronomer Edwin Hubble identified the diffuse elliptical and spiral objects that had been observed as galaxies. He went on to discover and measure a roughly linear relationship between the distance of these galaxies from Earth and their Doppler shift. In any direction one looks, the farther the galaxy appears, the faster it is receding from Earth.
Spectroscopic evidence that the universe was expanding was followed by the discovery in 1965 of a low level of isotropic microwave radiation by the American scientists Arno A. Penzias and Robert W. Wilson. The measured spectrum is identical to the radiation distribution expected from a blackbody, a surface that can absorb all the radiation incident on it. This radiation, which is currently at a temperature of 2.73 kelvin (K), is identified as a relic of the big bang that marks the birth of the universe and the beginning of its rapid expansion.