Spectroscopy in Astronomy
Electromagnetic radiation carries a lot of information about the nature of stars and other astronomical objects. To extract this information, however, astronomers must be able to study the amounts of energy we receive at different wavelengths of light in fine detail. Let’s examine how we can do this and what we can learn.
Properties of Light
Light exhibits certain behaviors that are important to the design of telescopes and other instruments. For example, light can be reflected from a surface. If the surface is smooth and shiny, as with a mirror, the direction of the reflected light beam can be calculated accurately from knowledge of the shape of the reflecting surface. Light is also bent, or refracted, when it passes from one kind of transparent material into another—say, from the air into a glass lens.
Reflection and refraction of light are the basic properties that make possible all optical instruments (devices that help us to see things better)—from eyeglasses to giant astronomical telescopes. Such instruments are generally combinations of glass lenses, which bend light according to the principles of refraction, and curved mirrors, which depend on the properties of reflection. Small optical devices, such as eyeglasses or binoculars, generally use lenses, whereas large telescopes depend almost entirely on mirrors for their main optical elements. We will discuss astronomical instruments and their uses more fully in Astronomical Instruments. For now, we turn to another behavior of light, one that is essential for the decoding of light.
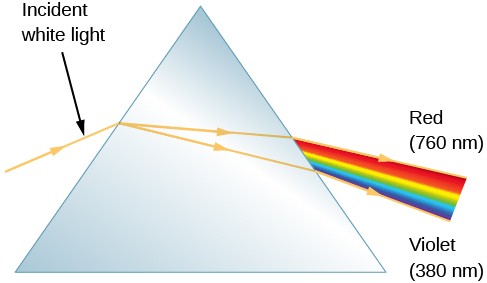
In 1672, in the first paper that he submitted to the Royal Society, Sir Isaac Newton described an experiment in which he permitted sunlight to pass through a small hole and then through a prism. Newton found that sunlight, which looks white to us, is actually made up of a mixture of all the colors of the rainbow (Figure 1).

Figure 1. Action of a Prism: When we pass a beam of white sunlight through a prism, we see a rainbow-colored band of light that we call a continuous spectrum.
Figure 1 shows how light is separated into different colors with a prism—a piece of glass in the shape of a triangle with refracting surfaces. Upon entering one face of the prism, the path of the light is refracted (bent), but not all of the colors are bent by the same amount. The bending of the beam depends on the wavelength of the light as well as the properties of the material, and as a result, different wavelengths (or colors of light) are bent by different amounts and therefore follow slightly different paths through the prism. The violet light is bent more than the red. This phenomenon is called dispersion and explains Newton’s rainbow experiment.
Upon leaving the opposite face of the prism, the light is bent again and further dispersed. If the light leaving the prism is focused on a screen, the different wavelengths or colors that make up white light are lined up side by side just like a rainbow (Figure 2). (In fact, a rainbow is formed by the dispersion of light though raindrops; see Note: The Rainbow feature box.) Because this array of colors is a spectrum of light, the instrument used to disperse the light and form the spectrum is called a spectrometer.
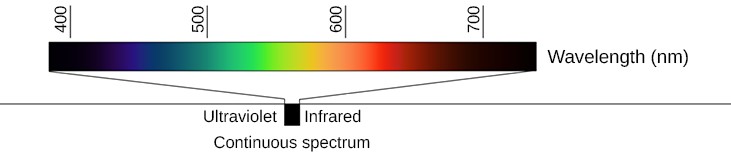
Figure 2. Continuous Spectrum: When white light passes through a prism, it is dispersed and forms a continuous spectrum of all the colors. Although it is hard to see in this printed version, in a well-dispersed spectrum, many subtle gradations in color are visible as your eye scans from one end (violet) to the other (red).
The Value of Stellar Spectra
When Newton described the laws of refraction and dispersion in optics, and observed the solar spectrum, all he could see was a continuous band of colors. If the spectrum of the white light from the Sun and stars were simply a continuous rainbow of colors, astronomers would have little interest in the detailed study of a star’s spectrum once they had learned its average surface temperature. In 1802, however, William Wollaston built an improved spectrometer that included a lens to focus the Sun’s spectrum on a screen. With this device, Wollaston saw that the colors were not spread out uniformly, but instead, some ranges of color were missing, appearing as dark bands in the solar spectrum. He mistakenly attributed these lines to natural boundaries between the colors. In 1815, German physicist Joseph Fraunhofer, upon a more careful examination of the solar spectrum, found about 600 such dark lines (missing colors), which led scientists to rule out the boundary hypothesis (Figure 3).
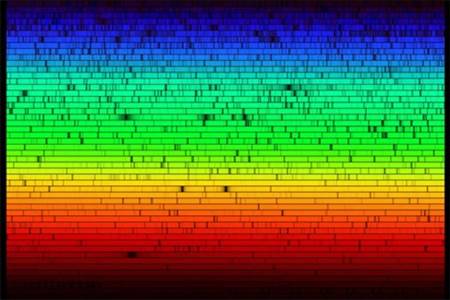
Figure 3. Visible Spectrum of the Sun: Our star’s spectrum is crossed by dark lines produced by atoms in the solar atmosphere that absorb light at certain wavelengths. (credit: modification of work by Nigel Sharp, NOAO/National Solar Observatory at Kitt Peak/AURA, and the National Science Foundation)
Later, researchers found that similar dark lines could be produced in the spectra (“spectra” is the plural of “spectrum”) of artificial light sources. They did this by passing their light through various apparently transparent substances—usually containers with just a bit of thin gas in them.
These gases turned out not to be transparent at all colors: they were quite opaque at a few sharply defined wavelengths. Something in each gas had to be absorbing just a few colors of light and no others. All gases did this, but each different element absorbed a different set of colors and thus showed different dark lines. If the gas in a container consisted of two elements, then light passing through it was missing the colors (showing dark lines) for both of the elements. So it became clear that certain lines in the spectrum “go with” certain elements. This discovery was one of the most important steps forward in the history of astronomy.
What would happen if there were no continuous spectrum for our gases to remove light from? What if, instead, we heated the same thin gases until they were hot enough to glow with their own light? When the gases were heated, a spectrometer revealed no continuous spectrum, but several separate bright lines. That is, these hot gases emitted light only at certain specific wavelengths or colors.
When the gas was pure hydrogen, it would emit one pattern of colors; when it was pure sodium, it would emit a different pattern. A mixture of hydrogen and sodium emitted both sets of spectral lines. The colors the gases emitted when they were heated were the very same colors as those they had absorbed when a continuous source of light was behind them. From such experiments, scientists began to see that different substances showed distinctive spectral signatures by which their presence could be detected (Figure 4). Just as your signature allows the bank to identify you, the unique pattern of colors for each type of atom (its spectrum) can help us identify which element or elements are in a gas.
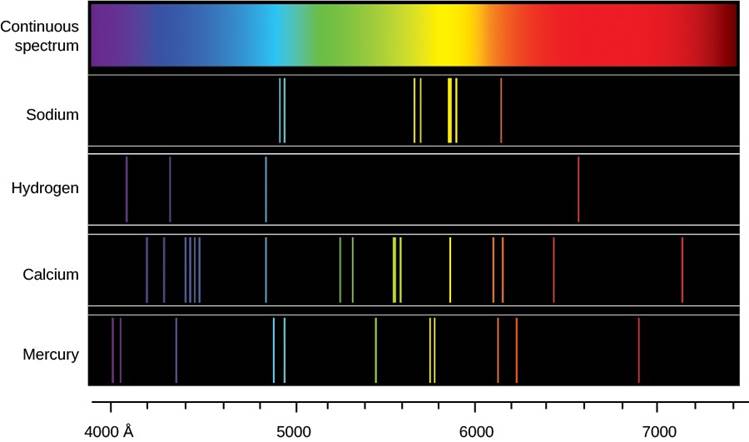
Figure 4. Continuous Spectrum and Line Spectra from Different Elements: Each type of glowing gas (each element) produces its own unique pattern of lines, so the composition of a gas can be identified by its spectrum. The spectra of sodium, hydrogen, calcium, and mercury gases are shown here.