Spectroscopy
Spectroscopy is the technique of splitting light (or more precisely electromagnetic radiation) into its constituent wavelengths (a spectrum), in much the same way as a prism splits light into a rainbow of colours. However, in general, a spectrum is generally more than a simple ‘rainbow’ of colours. The energy levels of electrons in atoms and molecules are quantised, and the absorption and emission of electromagnetic radiation only occurs at specific wavelengths. Consequently, spectra are not smooth but punctuated by ‘lines’ of absorption or emission.
Old style spectroscopy was carried out using a prism and photographic plates. These days, modern spectroscopy uses diffraction gratings to disperse the light, which is then projected onto CCDs (Charge Coupled Devices) similar to those used in digital cameras. The 2-dimensional spectra are easily extracted from this digital format and manipulated to produce 1-dimensional spectra like the galaxy spectrum shown below.
These 1-dimensional spectra contain an astonishing amount of useful data:

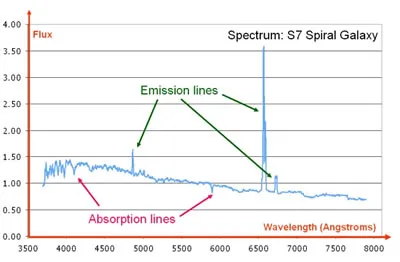
The spectrum of an S7 spiral galaxy showing both emission and absorption line features. Wavelength is measured in angstroms while the flux is in arbitrary units.
Credit: VizieR catalogue III/219, Spectral Library of Galaxies, Clusters and Stars (Santos et al. 2002)
The enormous quantities of information contained within a single spectrum makes spectroscopy one of the most powerful tools at an astronomer’s disposal.