Matric Potential
Matric potential arises because water is attracted to most surfaces through hydrogen bonding and van der Waals forces. This water droplet is pure but no longer free. The matric forces that bind it to the plastic have lowered its potential and you would have to use some energy to remove it from the surface and take it to a pool of pure, free water.
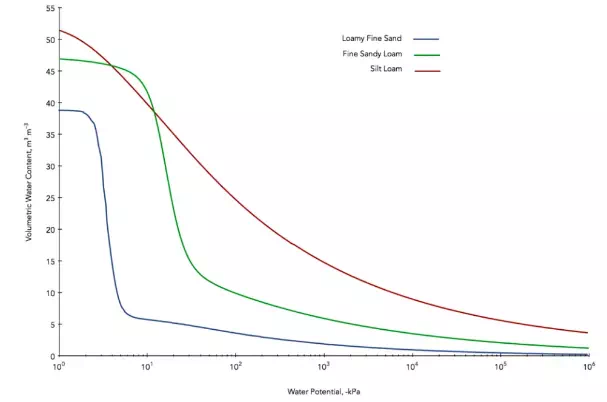
Soil is made up of small particles, providing lots of surfaces that will bind water. This binding is highly dependent on soil type. For example, sandy soil has large particles which provide less surface binding sites, while a silt loam has smaller particles and more surface binding sites.
The following figure showing moisture release curves for three different types of soil demonstrates the effect of surface area. Sand containing 10% water has a high matric potential, and the water is readily available to organisms and plants. Silt loam containing 10% water will have a much lower matric potential, and the water will be significantly less available.
Matric potential is always negative or zero, and is the most significant component of soil water potential in unsaturated conditions.