Rocket Science 101: Fuel, engine and nozzle
This is the
third in a series of posts on rocket science. Part I covered the
history of rocketry and Part II dealt with the operating principles
of rockets. If you have not checked out the latter post, I highly recommend you
read this first before diving into what is to follow.
We have
established that designing a powerful rocket means suspending a bunch of highly
reactant chemicals above an ultralight means of combustion. In terms of metrics
this means that a rocket scientist is looking to
● Maximise the mass ratio
to achieve the highest amounts of delta-v. This translates to carrying the
maximum amount of fuel with minimum supporting structure to maximise the
achievable change in velocity of the rocket.
● Maximise the specific
impulse of the propellant. The higher the specific impulse of the fuel the
greater the exhaust velocity of the hot gases and consequently the greater the
momentum thrust of the engine.
● Optimise the shape of
the exhaust nozzle to produce the highest amounts of pressure thrust.
● Optimise the staging
strategy to reach a compromise between the upside of staging in terms of
shedding useless mass and the downside of extra technical complexity involved
in joining multiple rocket engines (such complexity typically adds mass).
● Minimise the dry
mass costs of the rocket either by manufacturing simple expendable rockets at
scale or by building reusable rockets.
These
operational principles set the landscape of what type of rocket we want to
design. In designing chemical rockets some of the pertinent questions we need
to answer are
● What propellants to use
for the most potent reaction?
● How to expel and direct
the exhaust gases most efficiently?
● How to minimise the mass
of the structure?
Here, we will
turn to the propulsive side of things and answer the first of these two
questions.
Propellant
In a chemical
rocket an exothermic reaction of typically two different chemicals is used to
create high-pressure gases which are then directed through a nozzle and
converted into a high-velocity directed jet.
From
the Tsiolkovsky rocket equation we know
that the momentum thrust depends on the mass flow rate of the propellants and
the exhaust velocity,
![]()
The most common types of propellant are:
● Monopropellant: a single
pressurised gas or liquid fuel that disassociates when a catalyst is
introduced. Examples include hydrazine, nitrous oxide and hydrogen peroxide.
● Hypergolic propellant:
two liquids that spontaneously react when combined and release energy without
requiring external ignition to start the reaction.
● Fuel and oxidiser
propellant: a combination of two liquids or two solids, a fuel and an oxidiser,
that react when ignited. Combinations of solid fuel and liquid oxidiser are
also possible as a hybrid propellant system. Typical fuels include liquid
hydrogen and kerosene, while liquid oxygen and nitric acid are often used as
oxidisers. In liquid propellant rockets the oxidiser and fuel are typically
stored separately and mixed upon ignition in the combustion chamber, whereas
solid propellant rockets are designed premixed.
Rockets can
of course be powered by sources other than chemical reactions. Examples of this
are smaller, low performance rockets such as attitude control thruster, that
use escaping pressurised fluids to provide thrust. Similarly, a rocket may be
powered by heating steam that then escapes through a propelling nozzle.
However, the focus here is purely on chemical rockets.
Solid propellants
Solid
propellants are made of a mixture of different chemicals that are blended into
a liquid, poured into a cast and then cured into a solid. At its simplest,
these chemical blends or “composites” are comprised of four different
functional ingredients:
● Solid oxidiser granules.
● Flakes or powders of exothermic compounds.
● Polymer binding agent.
● Additives to stabilise or modify the burn rate.
Gunpowder is
an example of a solid propellant that does not use a polymer binding agent to
hold the propellant together. Rather the charcoal fuel and potassium nitrate
oxidiser are compressed to hold their shape. A popular solid rocket fuel is
ammonium perchlorate composite propellant (APCP) which uses a mixture of 70%
granular ammonium perchlorate as an oxidiser, with 20% aluminium powder as a
fuel, bound together using 10% polybutadiene acrylonitrile (PBAN).

Solid propellant rocket components (via Wikimedia Commons URL)
Solid
propellant rockets have been used much less frequently than liquid fuel
rockets. However, there are some advantages, which can make solid propellants
favourable to liquid propellants in some military applications (e.g.
intercontinental ballistic missiles, ICBMs). Some of the advantages of solid
propellants are that:
● They are easier to store
and handle.
● They are simpler to
operate with.
● They have less
components. There is no need for a separate combustion chamber and turbo pumps
to pump the propellants into the combustion chamber. The solid propellant (also
called “grain”) is ignited directly in the propellant storage casing.
● They are much denser
than liquid propellants and therefore reduce the fuel tank size (lower mass).
Furthermore, solid propellants can be used as a load-bearing component, which
further reduces the structural weight of the rocket. The cured solid propellant
can readily be encased in a filament-wound composite rocket shell, which has
more favourable strength-to-weight properties of the metallic rocket shells
typically used for liquid rockets.
Apart from
their use as ICBMs, solid rockets are known for their role as boosters. The
simplicity and relatively low cost compared with liquid-fuel rockets means that
solid rockets are a better choice when large amounts of cheap additional thrust
is required. For example, the Space Shuttle used two solid rocket boosters to complement
the onboard liquid propellant engines.
The
disadvantage of solid propellants is that their specific impulse, and hence the
amount of thrust produced per unit mass of fuel, is lower than for liquid
propellants. The mass ratio of solid rockets can actually be greater than that
of liquid rockets as a result of the more compact design and lower structural
mass, but the exhaust velocities are much lower. The combustion process in
solid rockets depends on the surface area of the fuel, and as such any air
bubbles, cracks or voids in the solid propellant cast need to be prevented.
Therefore, quite expensive quality assurance measures such as ultrasonic
inspection or x-rays are required to assure the quality of the cast. The second
problem with air bubbles in the cast is that the amount of oxidiser is
increased (via the oxygen in the air) which results in local temperature hot
spots and increased burn rate. Such local imbalances can spiral out of control
to produce excessive temperatures and pressures, and ultimately lead to
catastrophic failure. Another disadvantage of solid propellants are their
binary operation mode. Once the chemical reaction has started and the engines
have been ignited, it is very hard to throttle back or control the reaction.
The propellant can be arranged in a manner to provide a predetermined thrust
profile, but once this has started it is much hard to make adjustments on the
fly. Liquid propellant rockets on the other hand use turbo pumps to throttle
the propellant flow.
Liquid propellants
Liquid
propellants have more favourable specific impulse measures than solid rockets.
As such they are more efficient at propelling the rocket for a unit mass of
reactant mass. This performance advantage is due to the superior oxidising
capabilities of liquid oxidisers. For example, traditional liquid oxidisers
such as liquid oxygen or hydrogen peroxide result in higher specific impulse
measures than the ammonium perchlorate in solid rockets. Furthermore, as the
liquid fuel and oxidiser are pumped into the combustion chamber, a
liquid-fuelled rocket can be throttled, stopped and restarted much like a car
or a jet engine. In liquid-fuelled rockets the combustion process is restricted
to the combustion chamber, such that only this part of the rocket is exposed to
the high pressure and temperature loads, whereas in solid-fuelled rockets the
propellant tanks themselves are subjected to high pressures. Liquid propellants
are also cheaper than solid propellants as they can be sourced from the upper
atmosphere and require relatively little refinement compared to the composite
manufacturing process of solid propellants. However, the cost of the propellant
only accounts for around 10% of the total cost of the rocket and therefore
these savings are typically negligible. Incidentally, the high proportion of
costs associated with the structural mass of the rocket is why re-usability of
rocket stages is such an important factor in reducing the cost of spaceflight.
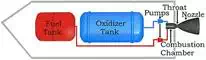
Schematic of a liquid-fuelled rocket (via Wikimedia Commons)
The main
drawback of liquid propellants is the difficulty of storage. Traditional liquid
oxidisers are highly reactive and very toxic such that they need to be handled
with care and properly insulated from other reactive materials. Second, the
most common oxidiser, liquid oxygen, needs to be stored at very low cryogenic
temperatures and this increases the complexity of the rocket design. What is
more, additional components such as turbopumps and
the associated valves and seals are needed that are entirely absent from
solid-fuelled rockets.
Modern spaceflight is dominated by two liquid propellant mixtures:
1. Liquid
oxygen (LOX) and kerosene (RP-1): As discussed in the previous post this
mix of oxidiser and fuel is predominantly used for lower stages (i.e. to get
off the launch pad), due to the higher density of kerosene compared to liquid
hydrogen. Kerosene, as a higher density fuel, allows for better ratios of
propellant to tankage mass which is favourable for the mass ratio. Second, high
density fuels work better in an atmospheric pressure environment. Historically,
the Atlas V, Saturn V and Soyuz rockets have used LOX and RP-1 for the first
stages and so does the SpaceX Falcon rocket today.
2. Liquid
oxygen and liquid hydrogen: This combination is mostly used for the upper
stages that propel a vehicle into orbit. The lower density of the liquid
hydrogen requires higher expansion ratios (gas pressure – atmospheric pressure)
and therefore works more efficiently at higher altitudes. The Atlas V, Saturn V
and modern Delta family or rockets all used this propellant mix for the upper
rocket stages.
The choice of propellant mixture for different stages requires
certain tradeoffs. Liquid hydrogen provides
higher specific impulse than kerosene, but its density is around 7 times lower
and therefore liquid hydrogen occupies much more space for the same mass of
fuel. As a result, the required volume and associated mass of tankage, fuel
pumps and pipes is much greater. Both the the specific
impulse of the propellant and tankage mass influence the potential delta-v of
the rocket, and hence liquid hydrogen, chemically the more efficient fuel, is
not necessarily the best option for all rockets.
Although the exact choice of fuel is not straightforward I will propose
two general rules of thumb that explain why kerosene is used for the early
stages and liquid hydrogen for the upper stages:
1. In
general, the denser the fuel the heavier the rocket on the launch pad. This
means that the rocket needs to provide more thrust to get off the ground and it
carries this greater amount of thrust throughout the entire duration of the
burn. As fuel is being depleted, the greater thrust of denser fuel rockets
means that the rocket reaches orbit earlier and as a result minimises drag
losses in the atmosphere.
2. Liquid
hydrogen fuelled rockets generally produce the lightest design and are
therefore used on those parts of the spacecraft that actually need to be
propelled into orbit or escape Earth’s gravity to venture into deep space.
Engine and Nozzle
In combustive
rockets, the chemical reaction between the fuel and oxidiser creates a high
temperature, high pressure gas inside the combustion chamber. If the combustion
chamber were closed and symmetric, the internal pressure acting on the chamber
walls would cause equal force in all directions and the rocket would remain
stationary. For anything interesting to happen we must therefore open one end of
the combustion chamber to allow the hot gases to escape. As a result of the hot
gases pressing against the wall opposite to the opening, a net force in the
direction of the closed end is induced.

Net thrust produced by rocket (via Wikimedia Commons)
Rocket
pioneers, such as Goddard, realised early on that the shape of the nozzle is of
crucial importance in creating maximum thrust. A converging nozzle
accelerates the escaping gases by means of the conservation of mass. However,
converging nozzles are fundamentally limited to fluid flows of Mach 1, the
speed of sound, and this is known as the choke condition. In this case, the
nozzle provides relatively little thrust and the rocket is purely propelled by
the net force acting on the close combustion chamber wall.
To further accelerate
the flow, a divergent nozzle is required at the choke point. A
convergent-divergent nozzle can therefore be used to create faster fluid flows.
Crucially, the Tsiolkovsky rocket equation
(conservation of momentum) indicates that the exit velocity of the hot gases is
directly proportional to the amount of thrust produced. A second advantage is
that the escaping gases also provide a force in the direction of flight by
pushing on the divergent section of the nozzle.

Underexpanded, perfectly expanded, over
expanded and grossly overexpanded de Laval
nozzles (via Wikimedia Commons).
The exit
static pressure of the exhaust gases, i.e. the pressure of the gases if the
exhaust jet was brought to rest, is a function of the pressure created inside
the combustion chamber and the ratio of throat area to exit area of the nozzle.
If the exit static pressure of the exhaust gases is greater than the
surrounding ambient air pressure, the nozzle is known to be underexpanded. On the other hand, if the exit static
pressure falls below the ambient pressure then the nozzle is known to be overexpanded. In this case two possible scenarios are
possible. The supersonic air flow exiting the nozzle will induce a shock wave
at some point along the flow. As the exhaust gas particles travel at speeds
greater than the speed of sound, other gas particles upstream cannot “get out
of the way” quickly enough before the rest of the flow arrives. Hence, the
pressure progressively builds until at some point the properties of the fluid,
density, pressure, temperature and velocity, change instantaneously. Thus,
across the shock wave the gas pressure of an overexpandednozzle
will instantaneously shift from lower than ambient to exactly ambient pressure.
If shock waves, visible by shock diamonds, form outside the nozzle, the nozzle
is known as simply overexpanded. However,
if the shock waves form inside the nozzle this is known asgrossly overexpanded.
In an ideal
world a rocket would continuously operate at peak efficiency, the condition
where the nozzle is perfectly expanded throughout the entire flight. This can
intuitively be explained using the rocket thrust equation introduced in
the previous post:
![]()
Peak
efficiency of the rocket engine occurs when ![]() such that the
pressure thrust contribution is equal to zero. This is the condition of peak
efficiency as the contribution of the momentum thrust is maximised while
removing any penalties from over- orunderexpanding the
nozzle. An underexpanded nozzle means
that
such that the
pressure thrust contribution is equal to zero. This is the condition of peak
efficiency as the contribution of the momentum thrust is maximised while
removing any penalties from over- orunderexpanding the
nozzle. An underexpanded nozzle means
that ![]() , and while this
condition provides extra pressure thrust,
, and while this
condition provides extra pressure thrust, ![]() is lower and some of the energy that has gone into
combusting the gases has not been converted into kinetic energy. In anoverexpanded nozzle the pressure differential is
negative,
is lower and some of the energy that has gone into
combusting the gases has not been converted into kinetic energy. In anoverexpanded nozzle the pressure differential is
negative, ![]() . In this case,
. In this case, ![]() is fully developed but the overexpansion induces a drag
force on the rocket. If the nozzle is grossly overexpanded such
that a shock wave occurs inside the nozzle,
is fully developed but the overexpansion induces a drag
force on the rocket. If the nozzle is grossly overexpanded such
that a shock wave occurs inside the nozzle, ![]() may still be greater than
may still be greater than ![]() but the supersonic jet separates from
the divergent nozzle prematurely (see diagram below) such that
but the supersonic jet separates from
the divergent nozzle prematurely (see diagram below) such that ![]() decreases. In outer space
decreases. In outer space ![]() decreases and therefore the thrust
created by the nozzle increases. However,
decreases and therefore the thrust
created by the nozzle increases. However, ![]() is also decreasing as the flow separates earlier from
the divergent nozzle. Thus, some of the increased efficiency of reduced ambient
pressure is negated.
is also decreasing as the flow separates earlier from
the divergent nozzle. Thus, some of the increased efficiency of reduced ambient
pressure is negated.
A perfectly
expanded nozzle is only possible using a variable throat area or variable exit
area nozzle to counteract the ambient pressure decrease with gaining altitude.
As a result, fixed area nozzles become progressively underexpanded as
the ambient pressure decreases during flight, and this means most nozzles are
grossly overexpanded at takeoff. Some various exotic nozzles such as plug
nozzles, stepped nozzles and aerospikes have been proposed to adapt
to changes in ambient pressure and increasing thrust at higher altitudes. The
extreme scenario obviously occurs once the rocket has left the Earth’s atmosphere.
The nozzle is now so grossly overexpanded that
the extra weight of the nozzle structure outweighs any performance gained from
the divergent section.
Thus we can
see that just as in the case of the propellants the design of individual
components is not a straightforward matter and requires detailed tradeoffs between different configurations. This is
what makes rocket science such a difficult endeavour.