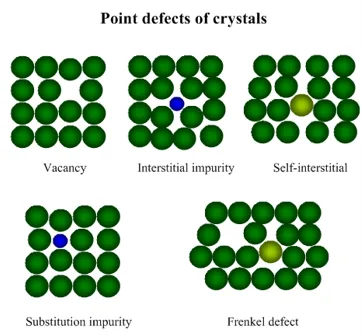
Imperfections of crystal structure
The simplest point defects are as follows:
§ Vacancy – missing atom at a certain crystal lattice position;
§ Interstitial impurity atom – extra impurity atom in an interstitial position;
§ Self-interstitial atom – extra atom in an interstitial position;
§ Substitution impurity atom – impurity atom, substituting an atom in crystal lattice;
§ Frenkel defect – extra self-interstitial atom, responsible for the vacancy nearby.

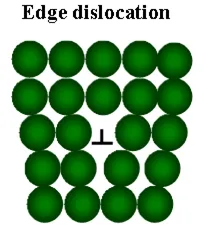
Linear crystal defects are edge and screw dislocations.
§ Edge dislocation is an extra half plane of atoms “inserted” into the crystal lattice. Due to the edge dislocations metals possess high plasticity characteristics: ductility and malleability.
§ Screw dislocation forms when one part of crystal lattice is shifted (through shear) relative to the other crystal part. It is called screw as atomic planes form a spiral surface around the dislocation line.
For quantitative characterization of a difference between a crystal distorted by a dislocation and the perfect crystal the Burgers vector is used.
The dislocation density is a total length of dislocations in a unit crystal volume. The dislocation density of annealed metals is about 1010 - 1012 m−². After work hardening the dislocation density increases up to 1015 - 1016 m-². Further increase of dislocation density causes crackes formation and fracture.
Planar defect is an imperfection in form of a plane between uniform parts of the material. The most important planar defect is a grain boundary. Formation of a boundary between two grains may be imagined as a result of rotation of crystal lattice of one of them about a specific axis. Depending on the rotation axis direction, two ideal types of a grain boundary are possible:
§ Tilt boundary – rotation axis is parallel to the boundary plane;
§ Twist boundary - rotation axis is perpendicular to the boundary plane:
§ An actual boundary is a “mixture” of these two ideal types.
Grain boundaries are called large-angle boundaries if misorientation of two neighboring grains exceeds 10º-15º.
Grain boundaries are called small-angle boundaries if misorientation of two neighboring grains is 5º or less.
Grains, divided by small-angle boundaries are also called subgrains.
Grain boundaries accumulate crystal lattice defects (vacancies, dislocations) and other imperfections, therefore they effect on the metallurgical processes, occurring in alloys and their properties.
Since the mechanism of metal deformation is a motion of crystal dislocations through the lattice, grain boundaries, enriched with dislocations, play an important role in the deformation process.
Diffusion along grain boundaries is much faster, than throughout the grains.
Segregation of impurities in form of precipitating phases in the boundary regions causes a form of corrosion, associated with chemical attack of grain boundaries. This corrosion is called Intergranular corrosion.