Atomic Structure of Solid Materials:
An atom of any element consists of the nucleus and the electrons. The nucleus is a stationary mass, situated at the centre and carrying a net positive charge. It consists of heavy particles—protons and neutrons. Each of these particles is 1836 times heavier than an electron. Each proton carries a positive charge while the neutron carries no charge.
The electrons revolve around the nucleus in definite orbits. They are bound to the nucleus by different energy levels. An electron has a very small mass, and carries a net negative charge. In its normal state an atom carries equal number of electrons and protons, and is therefore electrically neutral. Electrons belonging to an atom may be classified as inner and outer electrons.
The outer electrons are those which are least firmly bound to the atomic nucleus and revolve in the outer orbitals. They are also called valence electrons. The inner ones are more firmly bound to the atomic nucleus and revolve in the inner orbits. They are also called the core electrons. Since the valence electrons are not bounded firmly by the nucleus they can be easily removed from the atom. When this happens, the atom becomes a positive ion.
Each electron performs two types of motion simultaneously. One motion is revolution around and nucleus in definite orbits. The other is spinning on its own axis. The electrons never fall into the nucleus by the electro-static attraction between the two differently charged particles, and are held in their own orbits. This is because the electrostatic force of attraction is balanced by the kinetic energy of rotation and the potential energy of the electron.
In an atom the number of electrons in a given orbit and the motion they execute are fixed. Pauli discovered that a single electronic orbit can contain no more than two electrons at the same time, and that these two electrons must spin in opposite directions. Thus in all elements, whose atomic numbers are greater than 2, there exists more than one orbit.
A moving particle has wave properties associated with it. Thus a moving electron is associated with a wavelength given by the de-Broglie equation-
λ = h/p = h/mv
where, λ = Wave length of the moving electron
p = Momentum of the electron
m = Mass of the electron
v = Velocity of the electron
h = Planck’s constant.
Later, Einstein found that a definite packet of energy, called one quantum of energy, is involved in a unit atomic process. The magnitude of a quantum of energy is given by-
E = hv
Where,
E = Magnitude of a quantum of energy
v = Frequency of radiation
The equation states that there is one to one relationship between the frequency of radiation and the magnitude of energy packets. The frequency of radiation is related to its wavelength by the following equation-
v = c/λ
where,
c = Velocity of light
λ = Wavelength of radiation.
Energy Levels and Quantum Numbers:
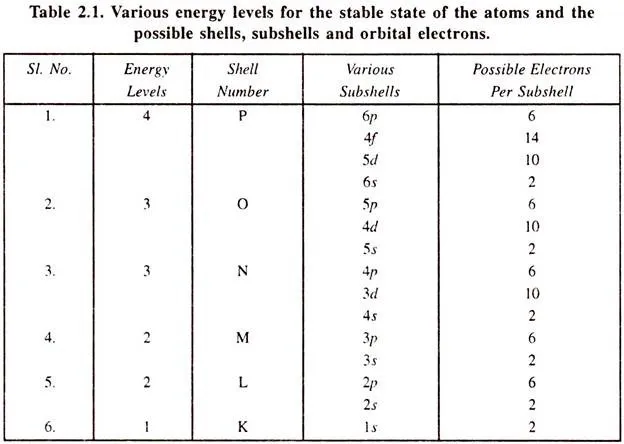
The electrons in an atom are arranged in different shells, known as K-shell, L-shell, M-shell, etc. Each shell includes a fixed number of orbits. These shells are further divided into subshells depending upon the total number of electrons in each shell. Each subshell (orbit) is at a definite distance and the nucleus exerts a definite force on the electrons in this subshell.
This force is known as the energy of the orbit. Each subshell which can be occupied by the electrons is called energy level in the atom. Each electron of the atom belongs to a particular orbit and hence occupies a definite energy level. The total energy of the atom is the sum of the energy levels occupied by different electrons.
The arrangement of electrons in different orbits is such that it produces a minimum total energy in the atom. This arrangement of electrons becomes the most stable state of the atom. If an electron occupies a higher energy orbit, it is called an excited state of the atom.
Various shells, the subshells and the number of electrons which can occupy a given subshell are shown in F 2.1.
Each electron in the atom occupies a particular shell and subshell. The exact position of the electron in the atom is specified by quantum numbers. There are four quantum numbers. In an atom, no two electrons can have all the four quantum numbers same at any time.
These four quantum numbers are as follows:
1. Principal quantum number ‘n’ which is a measure of the energy of the main shell. The electron shells are designated as K, L, M, N, O … corresponding to value of principal quantum number n = 1, 2, 3, 4, 5 … respectively. The number of electrons in any shell is given by 2n2 e.g. the number of electrons in L-shell are 8 only.
2. Second quantum number, ‘l’ which is a measure of the angular momentum of the electron. The value of ‘l’ is useful to calculate the maximum number of electrons in a subshell which is equal to (2l + 1) e.g. the number of electrons in a sub-shell corresponding to l = 0 are 2. Moreover, the sub-shells in each main shell are designated by small alphabets s, p, d, f … corresponding to quantum number ‘l’ = 0, 1, 2, 3, … respectively.
3. Third quantum number ‘m1‘, which is a measure of the component of the angular momentum in particular direction.
4. The fourth quantum number, ‘ms‘ which determines the direction of spinning of the electron.
An electron having all the four different quantum numbers constitutes a quantum state. The various quantum states of the electrons in the first three shells of the atom are shown in the Table 2.2.