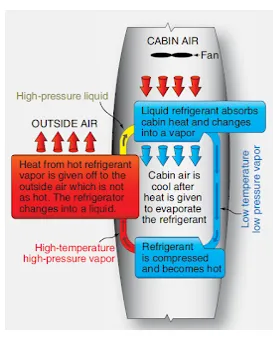
Figure 10. In vapor cycle air conditioning, heat is carried from the cabin to the outside air by a refrigerant which changes from a liquid to a vapor and back again
Vapor Cycle Air Conditioning
The absence of a bleed air source on reciprocating engine aircraft makes the use of an air cycle system impractical for conditioning cabin air. Vapor cycle air conditioning is used on most nonturbine aircraft that are equipped with air conditioning. However, it is not a source of pressurizing air as the air cycle system conditioned air is on turbine powered aircraft. The vapor cycle system only cools the cabin. If an aircraft equipped with a vapor cycle air conditioning system is pressurized, it uses one of the sources discussed in the pressurization section above. Vapor cycle air conditioning is a closed system used solely for the transfer of heat from inside the cabin to outside of the cabin. It can operate on the ground and in flight.
Theory of Refrigeration
Energy can be neither created nor destroyed; however, it can be transformed and moved. This is what occurs during vapor cycle air conditioning. Heat energy is moved from the cabin air into a liquid refrigerant. Due to the additional energy, the liquid changes into a vapor. The vapor is compressed and becomes very hot. It is removed from the cabin where the very hot vapor refrigerant transfers its heat energy to the outside air. In doing so, the refrigerant cools and condenses back into a liquid. The refrigerant returns to the cabin to repeat the cycle of energy transfer. [Figure 10]
|
Figure 10. In vapor cycle air conditioning, heat is carried from the cabin to the outside air by a refrigerant which changes from a liquid to a vapor and back again |
Heat is an expression of energy, typically measured by temperature. The higher the temperature of a substance, the more energy it contains. Heat always flows from hot to cold These terms express the relative amount of energy present in two substances. They do not measure the absolute amount of heat present. Without a difference in energy levels, there is no transfer of energy (heat).
Adding heat to a substance does not always raise its temperature. When a substance changes state, such as when a liquid changes into a vapor, heat energy is absorbed. This is called latent heat. When a vapor condenses into a liquid, this heat energy is given off. The temperature of a substance remains constant during its change of state. All energy absorbed or given off, the latent heat, is used for the change process. Once the change of state is complete, heat added to a substance raises the temperature of the substance. After a substance changes state into a vapor, the rise in temperature of the vapor caused by the addition of still more heat is called superheat.
The temperature at which a substance changes from a liquid into a vapor when heat is added is known as its boiling point. This is the same temperature at which a vapor condenses into a liquid when heat is removed. The boiling point of any substance varies directly with pressure. When pressure on a liquid is increased, its boiling point increases, and when pressure on a liquid is decreased, its boiling point also decreases. For example, water boils at 212 °F at normal atmospheric temperature (14.7 psi). When pressure on liquid water is increased to 20 psi, it does not boil at 212 °F. More energy is required to overcome the increase in pressure. It boils at approximately 226.4 °F. The converse is also true. Water can also boil at a much lower temperature simply by reducing the pressure upon it. With only 10 psi of pressure upon liquid water, it boils at 194 °F. [Figure 11]
|
Figure 11. Boiling point of water changes as pressure changes |
Vapor pressure is the pressure of the vapor that exists above a liquid that is in an enclosed container at any given temperature. The vapor pressure developed by various substances is unique to each substance. A substance that is said to be volatile, develops high vapor pressure at standard day temperature (59 °F). This is because the boiling point of the substance is much lower. The boiling point of tetrafluoroethane (R134a), the refrigerant used in most aircraft vapor cycle air conditioning systems, is approximately –15 °F. Its vapor pressure at 59 °F is about 71 psi. The vapor pressure of any substance varies directly with temperature.
Basic Vapor Cycle
Vapor cycle air conditioning is a closed system in which a refrigerant is circulated through tubing and a variety of components. The purpose is to remove heat from the aircraft cabin. While circulating, the refrigerant changes state. By manipulating the latent heat required to do so, hot air is replaced with cool air in the aircraft cabin.
To begin, R134a is filtered and stored under pressure in a reservoir known as a receiver dryer. The refrigerant is in liquid form. It flows from the receiver dryer through tubing to an expansion valve. Inside the valve, a restriction in the form of a small orifice blocks most of the refrigerant. Since it is under pressure, some of the refrigerant is forced through the orifice. It emerges as a spray of tiny droplets in the tubing downstream of the valve. The tubing is coiled into a radiator type assembly known as an evaporator. A fan is positioned to blow cabin air over the surface of the evaporator. As it does, the heat in the cabin air is absorbed by the refrigerant, which uses it to change state from a liquid to a vapor. So much heat is absorbed that the cabin air blown by the fan across the evaporator cools significantly. This is the vapor cycle conditioned air that lowers the temperature in the cabin.
The gaseous refrigerant exiting the evaporator is drawn into a compressor. There, the pressure and the temperature of the refrigerant are increased. The high-pressure high-temperature gaseous refrigerant flows through tubing to a condenser. The condenser is like a radiator comprised of a great length of tubing with fins attached to promote heat transfer. Outside air is directed over the condenser. The temperature of the refrigerant inside is higher than the ambient air temperature, so heat is transferred from the refrigerant to the outside air.
The amount of heat given off is enough to cool the refrigerant and to condense it back to a high-pressure liquid. It flows through tubing and back into the receiver dryer, completing the vapor cycle.
There are two sides to the vapor cycle air conditioning system. One accepts heat and is known as the low side. The other gives up heat and is known as the high side. The low and high refer to the temperature and pressure of the refrigerant. As such, the compressor and the expansion valve are the two components that separate the low side from the high side of the cycle. [Figure 12] Refrigerant on the low side is characterized as having low pressure and temperature. Refrigerant on the high side has high pressure and temperature.
|
Figure 12. A basic vapor cycle air conditioning system. The compressor and the expansion valve are the two components that separate the low side from the high side of the cycle. This figure illustrates this division. Refrigerant on the low side is characterized as having low pressure and temperature. Refrigerant on the high side has high pressure and temperature |